Table of Contents
FEM-Simulated ISO14801-tests can help compare and optimize products in the design phase. They can also substitute and supplement experimental tests, reducing the number of time-consuming Fatigue Tests necessary for medical product approval.
Experiments & Simulations
Experiments are necessary for getting a product approved for medical use. But experiments, such as fatigue testing, can be extremely time consuming, expensive and are not always very precise.
Simulations can be used as a supplement to experiments, or in some cases replace them completely. Furthermore, designs can be checked and optimized already in the R&D phase. This minimizes the risk of creating bad designs, that make it all the way to prototype or approval testing. Simulations can also reduce the number of experiments, by identifying worst case component configurations or by extrapolating existing data, for example using fatigue test simulation.
Simulated ISO14801
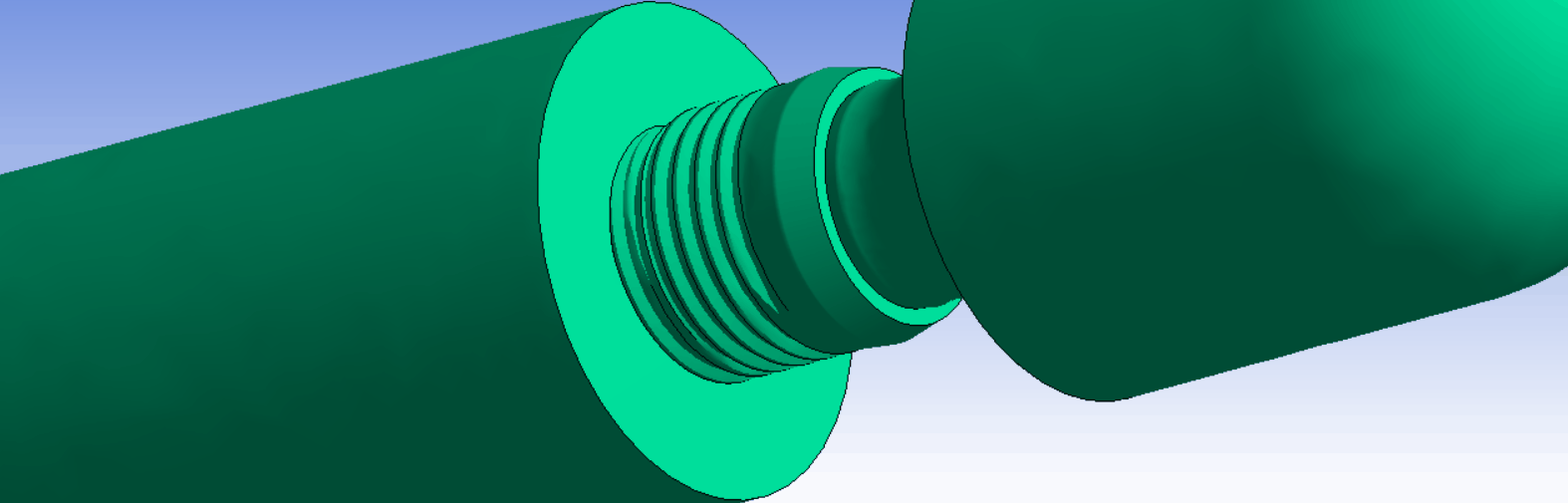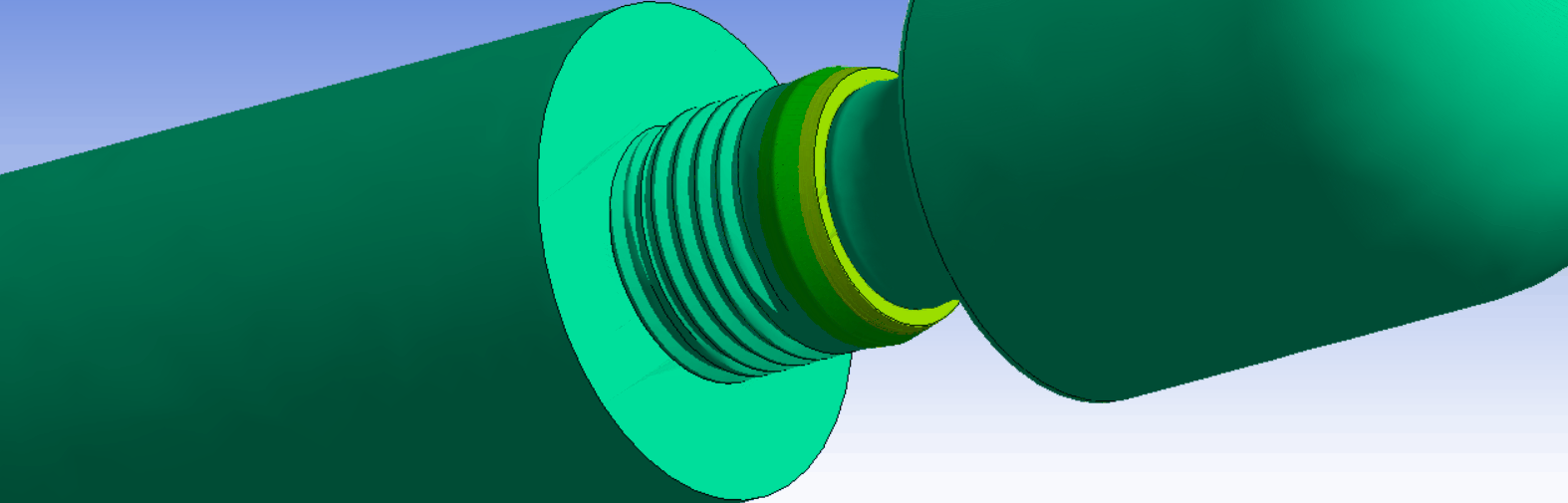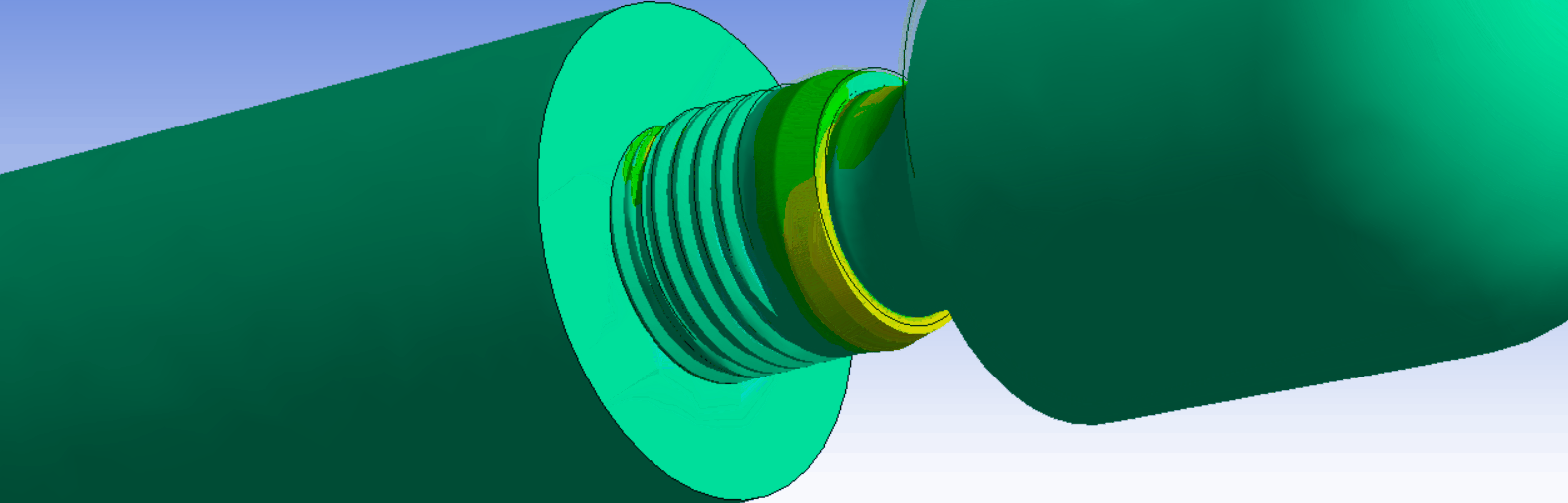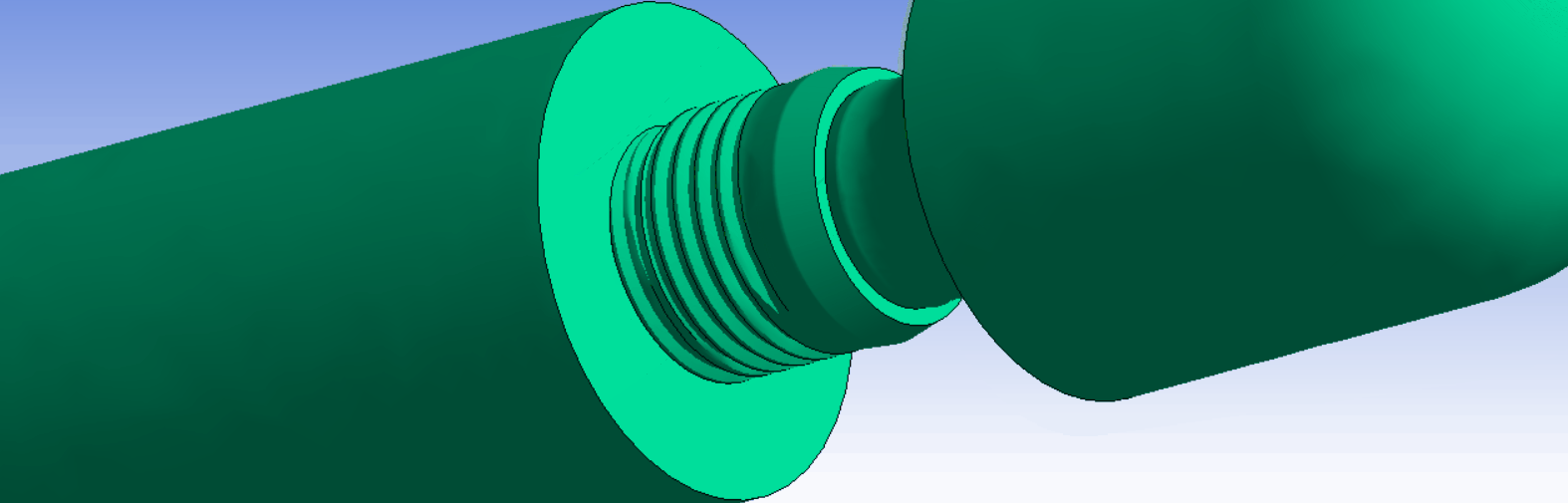
The ISO14801 test standard is the backbone of dental implant testing. It is used for both max-loading and fatigue tests. It specifies a standardized test setup, where the implant is fixated, and an angled external load is applied through a force cap. The standard works well as a basis for determining and comparing the strength of dental implant systems.
It is possible to exactly replicate the ISO14801 test in simulation. This is very useful, because it makes it possible to directly compare results between experiments and simulations. In addition, it is also helpful when comparing strength between different implant systems, design variations, or for optimizing production and design parameters.
(a) Forcecap for MUA 
(b) Implant fixture
Identifying Worst-Case
Identifying the worst-case component configurations can be a way to reduce the number of experiments necessary for product approval. A dental implant system consists of several components: Implants with different diameter and length, straight abutments, angled multi-unit abutments etc. All these parts need to be approved for medical use, but not every combination needs to be tested in experiments. In many cases, it is possible to get families of products medically approved by testing only a selection of components. This selection must contain the weakest combinations, most likely to fail. Mechanically similar, but arguably stronger combinations can then be approved by comparison.
However, to determine and justify that two parts are mechanically similar requires insight in their internal stress distribution mechanisms. For the same reason, it can be practically impossible to deduct which configurations to consider as worst case. If the wrong component configurations are selected, additional testing is required. This is expensive and can set back the approval process for months. Finite Element Analysis may be used to quickly and safely determine which components configurations should be tested, and what results to expect. Unnecessary experiments can be avoided, and the argumentation and documentation necessary for a successful medical approval is solid and readily available.
Simulated Fatigue Analysis
Most mechanical components display different strength in a single loading versus a cyclic loading scenario. Material fatigue is the process in which certain materials (including most metals) weaken and fail under repeated loading. Due to material fatigue, the product will typically be weaker in repeated loading, than in a single loading test. Fatigue testing is therefore very important for dental implants, as it gives a more accurate picture of how the implant system will perform over years of use.
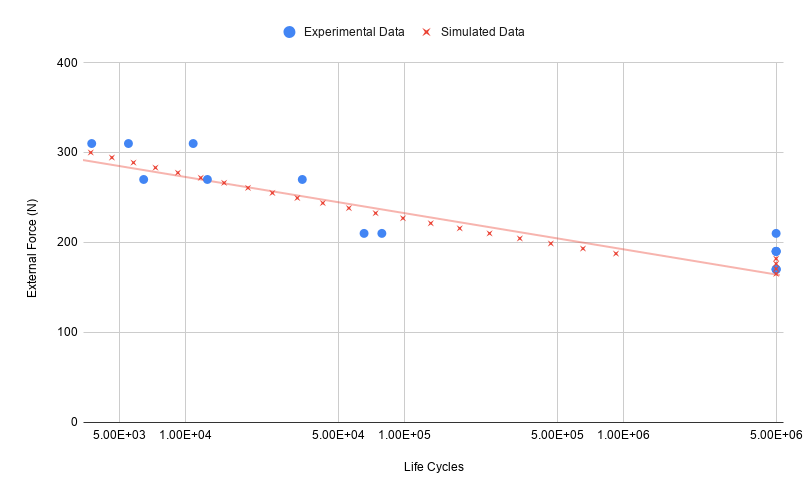
Due to the nature of material behavior under repeated loading, fatigue testing cannot be substantially accelerated. Consequently, fatigue tests must run uninterrupted for months and usually only include a small number of test-samples. In addition, fatigue-life is strongly influenced by production factors such as surface roughness and material imperfections. For implant companies, this means expensive tests that take months to complete and often yield results with extremely high statistical spread.
As a first-of-its-kind, Det Tekniske Bureau can quickly deliver precise and reliable fatigue results based on simulations. These can be used to supplement and extrapolate experimental data. They can also be used directly in the design phase to evaluate and optimize product performance under repeated loading.